FDA panel endorses Moderna’s COVID-19 booster vaccine for certain high risk groups
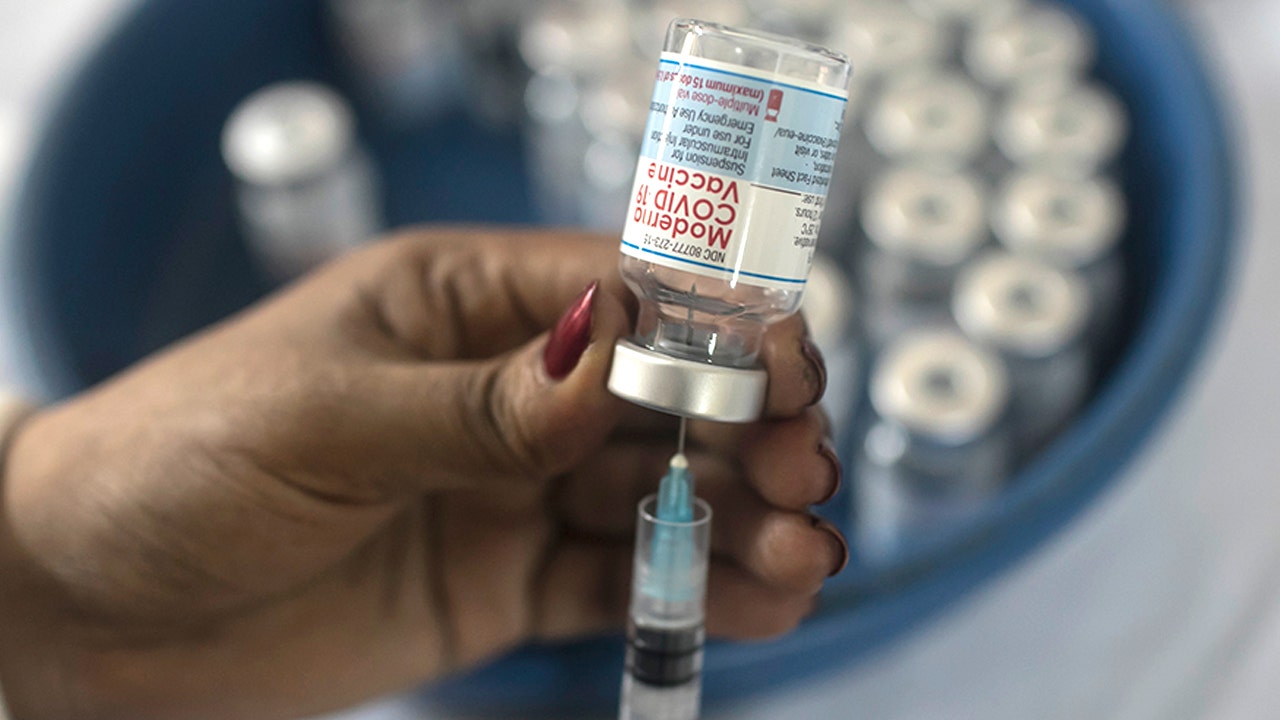
A U.S. Food and Drug Administration (FDA) advisory panel on Thursday endorsed emergency authorization for Moderna’s half-dose COVID-19 vaccine booster shot when administered at least six months following the two-dose series among people ages 65 and older and those ages 18-64 at high risk of occupational exposure and severe COVID-19.















No comments:
Post a Comment
Do not add any spam link on Comment Section.